
Background: The ENESTop study (NCT01698905) evaluates treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) with sustained deep molecular response following second-line (2L) treatment with nilotinib (NIL). In the primary analysis, 57.9% of pts remained in TFR 48 weeks after stopping NIL and previous analysis at ≥3.7 years showed durability of TFR. The present analysis first assessed long-term TFR outcomes at ≥5 years and then a post-hoc analysis evaluated rates of regain of molecular response following NIL re-initiation in pts with loss of major molecular response (MMR) or confirmed loss of MR4.0 at a follow-up of 5 years.
Methods: Eligible pts had received ≥3 years of treatment (including >4 weeks of imatinib followed by ≥2 years of NIL) and had achieved MR4.5 (BCR-ABL1IS ≤0.0032%) on NIL. Pts without confirmed loss of MR4.5 after 1 year of consolidation treatment (CONS) could attempt TFR. NIL was re-initiated upon loss of MMR (BCR-ABL1IS >0.1%) or confirmed loss of MR4.0 (BCR-ABL1IS >0.01% in 2 consecutive assessments). By data cut-off (29 Jan 2020) pts had completed ≥5 years of TFR, resumed NIL, or discontinued the study.
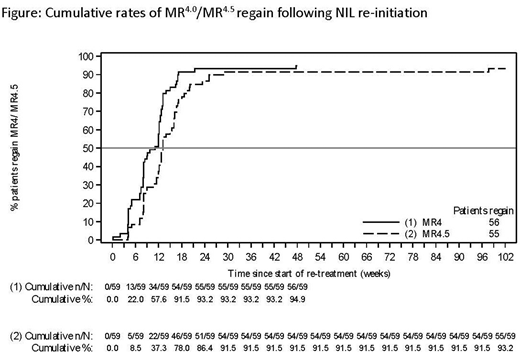
Results: At the data cut-off, of the 126 pts who entered TFR, 52 remained in TFR, 59 had resumed NIL and 15 had discontinued the study. At 5 years, the TFR rate was 42.9% (54/126; 95% CI, 34.1-52.0). Compared with pts in TFR at the end of the 3.7-year follow-up, an additional 4 pts were not in TFR at the 5-year follow-up, none due to loss of response: 2 pts discontinued TFR due to pt/guardian decision, 1 pt had an ischemic stroke and died in the post-treatment follow-up phase, and 1 pt was lost to follow-up. The MR4.5 rate at 5 years after starting TFR was 37.3% (47/126, 95% CI: 28.9-46.4). 11 pts with MR4.5 at 5 years had a temporary loss of MR4.5 while 36/126 (28.6%) maintained stable MR4.5 for 5 years. The estimated treatment-free survival (TFS) rate at 5 years was 49.4% (95% CI: 40.3-57.9). Of the 59 pts who resumed NIL, 58 (98.3%) regained MMR. The only pt who did not regain MMR stopped retreatment at 20 weeks and withdrew from the study. 56 (94.9%) pts regained MR4.0 and 55 (93.2%) regained MR4.5 (Figure). The median time to regain MR4.5 was 2.9 months (range: 0.9-22.5). Baseline characteristics between pts who resumed NIL due to loss of MMR (n=34) or confirmed loss of MR4.0 (n=25)were similar; more pts with loss of MMR had a low Sokal relative risk score at diagnosis (44.1% vs 16.0%) and pts with loss of MMR had a shorter median time from achievement of MR4.5 until TFR entry (27.9 vs 40.1 months). In pts with confirmed loss of MR4.0, 25/25 (100.0%) and 24/25 (96.0%) regained MR4.0 and MR4.5 within the first 48 weeks of NIL re-initiation, respectively. In pts with loss of MMR, 33/34 (97.1%), 31/34 (91.2%) and 30/34 (88.2%) regained MMR, MR4.0 and MR4.5 within the first 48 weeks of NIL re-initiation, respectively. The median time to regain MR4.5 for pts who resumed NIL due to confirmed loss of MR4.0 or loss of MMR was 2.0 months (range: 0.9-4.6) and 3.6 months (range: 1.6-22.5), respectively. There were no cases of disease progression or death due to underlying CML. For pts remaining in TFR >3.7 years (n=58), the rates of all-grade adverse events (AEs) were 50.0% in the fifth 48 weeks of TFR compared with 55.2% in the fourth 48 weeks. The musculoskeletal pain AE rate was high during the first 48 weeks of TFR (53.4%) but by the fifth 48 weeks of TFR was lower (6.9%) than during CONS (10.3%). In pts who resumed NIL (n=59), the rate of all-grade AEs was higher (94.9%) than during CONS (77.9%, n=163). Overall all-grade AE rates were similar between pts re-initiating NIL due to loss of MMR (94.1%) or confirmed loss of MR4.0 (96.0%), with the most common AEs being hypertension (26.5% vs 32.0%), arthralgia (20.6% vs 8.0%), constipation (11.8% vs 20.0%) and hyperglycemia (26.5% vs 0%).
Conclusions: These results illustrate the long-term (up to 5 years) durability and safety of TFR in pts with CML-CP following 2L NIL. The majority of pts requiring NIL re-treatment rapidly regained MMR, MR4.0 or MR4.5. The faster rate of regain in pts resuming NIL due to confirmed loss of MR4.0 likely reflects earlier intervention but could also suggest a slower tempo of relapse and possibly more favorable biology. Subgroup analysis should be interpreted with caution due to small base sizes. No new or unexpected safety signals were observed.
Mahon:ARIAD: Honoraria; Novartis Pharma: Honoraria, Research Funding; Pfizer: Honoraria; BMS: Honoraria. Clementino:EMS: Other: Financial support for congress. Fominykh:Pfizer: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau. Lipton:Takeda: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; Bristol-Myers Squibb: Honoraria. Turkina:Pfizer: Honoraria; Novartis Pharma: Honoraria; BMS: Honoraria. Moiraghi:Novartis: Speakers Bureau; BMS: Speakers Bureau. Nicolini:Sun Pharma Ltd: Consultancy; Incyte: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Takahashi:Pfizer Japan Inc.: Honoraria, Research Funding; Novartis Pharma KK: Honoraria, Research Funding; Bristol-Myers Squibb Company: Honoraria. Sacha:Incyte: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers Squibb Company: Consultancy, Honoraria, Speakers Bureau; Adamed: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau. Kim:Sun Pharma.: Research Funding; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; ILYANG: Consultancy, Honoraria, Research Funding; Takeda: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Fellague-Chebra:Novartis: Current Employment, Current equity holder in publicly-traded company, Research Funding. Tiwari:Novartis: Current Employment. Bouard:Novartis: Current Employment. Hughes:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal